- Home
- Health
- Water Softener Guide
- Water Treatment
Private Sewers & Septic Systems
- About Private Sewers Sysytem And Septic Systems
- Lateral Root Notification Program
- Fats, Oil & Grease
- Spills & Black Water Valves
- Help Protect The Enviroment
- Septic Systems
- About
Hard Water pH vs. Soft Water pH Levels

Understanding the pH levels of the water in our homes is essential for our health, appliances, and plumbing systems. Many people wonder about the nature of hard water and soft water, especially in terms of their pH levels.
Introduction
The question of whether hard water is acidic or alkaline comes up frequently.
To address this:
Soft water is Acidic, with pH levels below 7.0.
A Neutral pH is exactly 7.0
Hard water is Alkaline, meaning its pH levels are above 7.0.
Visual cues, such as red versus blue pH indicator paper, can quickly show the pH level of a water sample.
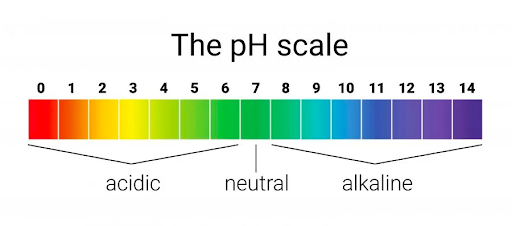
Understanding pH Levels
Knowing the pH level of your home’s water is crucial because it affects everything from skin health to the longevity of your plumbing.
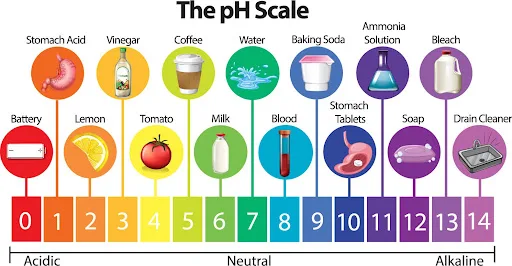
Hard Water Explained
Hard water is characterized by its high mineral content, particularly calcium and magnesium ions.
These minerals increase water’s alkalinity, hence why hard water typically has a pH level above 7.0.
The scientific basis for this lies in the dissolution of calcium carbonate (CaCO3) in water, which forms calcium ions (Ca2+) and hydroxide ions (OH−), making the water alkaline.
Soft Water Explained
In contrast, soft water has lower concentrations of calcium and magnesium, which means it generally falls below the neutral pH mark, making it acidic. This softness is often achieved through ion exchange or other water softening processes, which remove these hardness minerals.
Measuring Water Hardness and pH
Determining water hardness at home is straightforward with a pH kit. By measuring pH, you can infer water hardness: a higher pH (above 7.0) suggests hard water, while a lower pH indicates soft water. For example, a pH reading of 9.0 almost certainly points to hard water.

Water Hardness pH Chart
We’ve created a chart that correlates water hardness levels with pH, ranging from soft water (below 7.0 pH) to very hard water (8.5 pH or above). This chart helps users to visually match their water’s pH level with its probable hardness category.
PPM (Parts Per Million): | pH Level: |
0 to 30 PPM | Below 6.5 pH |
35 PPM | 6.6 pH |
40 PPM | 6.7 pH |
45 PPM | 6.8 pH |
50 PPM | 6.9 pH |
60 PPM | 7.0 pH |
70 PPM | 7.1 pH |
80 PPM | 7.1 pH |
90 PPM | 7.2 pH |
100 PPM | 7.3 pH |
110 PPM | 7.4 pH |
120 PPM | 7.5 pH |
130 PPM | 7.6 pH |
140 PPM | 7.8 pH |
150 PPM | 7.9 pH |
160 PPM | 8.1 pH |
170 PPM | 8.3 pH |
180 PPM | 8.5 pH |
190 PPM | 8.6 pH |
200 PPM | 8.7 pH |
250 PPM | 8.9 pH |
300 to 1000 PPM | Over 9.0 pH |
The Impact of Other Ions on Water pH
Water’s pH can also be influenced by other dissolved ions, such as sodium, iron, potassium, and magnesium. These elements can alter the water’s pH and, by extension, its perceived hardness, highlighting the complexity of water chemistry.
While pH provides a rough estimate of water hardness, more accurate methods exist, such as home water hardness kits. These tests offer a more precise measurement of water hardness, beyond just the alkaline or acidic nature indicated by pH.
Conclusion
To sum up, hard water is generally alkaline, while soft water tends to be acidic. Understanding and measuring the pH of your water can provide valuable insights into its quality and hardness.
If you have questions or want to share your results, feel free to comment below.
FAQ Section: Understanding pH in Hard and Soft Water
What pH is soft water?
Soft water typically has a pH level below 7.0, indicating it is acidic.
What is soft water pH?
The pH of soft water is generally below 7.0, classifying it as acidic.
What is the pH of soft water?
Soft water's pH is usually less than 7.0, reflecting its acidic nature.
Hard water pH levels:
Hard water has pH levels above 7.0, showing it is alkaline.
Soft water pH:
The pH of soft water falls under 7.0, meaning it is acidic.
Softened water pH:
Softened water often has a pH close to neutral but can vary depending on the method used for softening.
What is pH of hard water?
The pH of hard water is above 7.0, indicating alkalinity.
What is the pH of softened water?
The pH of softened water can slightly vary, usually hovering around neutral.
What pH is hard water?
Hard water's pH is typically greater than 7.0, indicating its alkaline nature.
Hard water pH level:
The pH level of hard water is above 7.0, signifying it is alkaline.
Is hard water alkaline or acidic?
Hard water is alkaline, with a pH above 7.0.
pH of hard water:
Hard water maintains a pH level above 7.0, showcasing its alkalinity.
pH soft water:
Soft water has a pH below 7.0, making it acidic.
Does hard water have a high pH?
Yes, hard water typically has a high pH, indicating its alkalinity.
Hard vs soft water pH:
Hard water is alkaline (pH > 7.0), while soft water is acidic (pH < 7.0).
Is hard water acidic?
No, hard water is not acidic; it is alkaline.
Is hard water acidic or alkaline?
Hard water is alkaline, with a pH level above 7.0.
pH water hardness:
Water hardness increases with alkalinity, so a higher pH suggests harder water.
Water hardness and pH:
There's a direct relationship; as water hardness increases, so does pH, making the water more alkaline.
Water hardness and pH relationship:
The relationship indicates that harder water typically has a higher pH, making it more alkaline.








